Alternative Energy Source — Methane Hydrate?
The article at the LLNL Web site estimates that the energy locked up in methane hydrate deposits is more than twice the global reserves of all conventional gas, oil, and coal deposits combined. Just as with the exploitation of nuclear energy, there are safety and economic issues associated with using this vast energy resource. The hydrate is essential an ice that can quickly melt and disassociate releasing methane gas. The LLNL is researching the properties of this "ice."
An immediate safety concern is that exploratory drilling for more common natural gas or petroleum deposits could release methane causing fires and explosions. At the worst, scenarios for catastrophes of global proportions exist. These global scenarios include massive releases of methane (a greenhouse gas) into the environment or even tsunamis from sudden large area methane releases as the result of earthquakes or undersea avalanches.
A Livermore-United States Geological Survey team developed an entirely new procedure to produce methane hydrate ice. They mixed granular water ice and cold, pressurized methane gas in a constant-volume vessel and slowly heated it. Warming started at a temperature of –10°F. The reaction between methane and water ice started just below the normal melting point of water ice (29°F) and continued until virtually all of the water ice had reacted with methane.
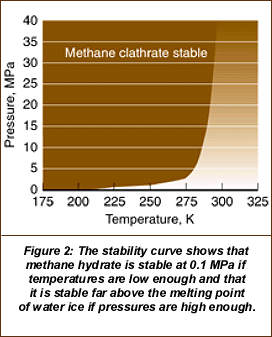
It worked, but some unexpected things happened. The water ice did not liquefy, as it should have when its melting temperature was reached and surpassed. In fact, the temperatures inside the reaction vessel reached 50°F (18 degrees above the melting point of water ice) before the last of the water ice melted. The ability to test methane hydrate ice is important in order to understand the risks presented in natural circumstances as well as the risks that could be associated with attempts to exploit (mine?) methane hydrate. The figures from the LLNL article shown below indicated a somewhat mixed picture. At high pressures (0.1 MPa equals 1 atmosphere or 14 pounds/square inch), the hydrate is stable even above the melting point of water (273 degrees K in the figures).
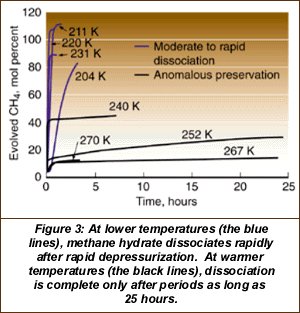
Oddly, the hydrate is more stable at temperatures around the melting point of water ice than at much cooler temperatures. [In Figure 3 below, 240° K is about 40° F below zero.]
Although methane is a greenhouse gas, the combustion of methane may be the best choice for fossil fuel energy resources. [Major natural sources of methane are the digestion processes of cattle and composting.] Coal is essentially 100% carbon and all of the combustion gases will be either carbon monoxide [CO] or carbon dioxide [CO2]. Combustion of methane [CH4] yields two water molecules [H2O] for every molecule of carbon dioxide.
Given the complex nature of the stability of methane hydrate, the need for high tech solutions for underwater recovery and the fact that international law will apply; eventual energy industry exploitation of this resource will likely be highly regulated. Just as with nuclear power:
- The potential resources are immense.
- Safety concerns are likely to be resolvable but with a very small remaining risk of a serious accident.
- Large capital investments in technology and facilities will be required.
- If accidental releases are avoided, the total energy fuel cycle is friendly to the environment.
Eventually, there will likely be both substantial nuclear energy and methane hydrate in our energy mix.
